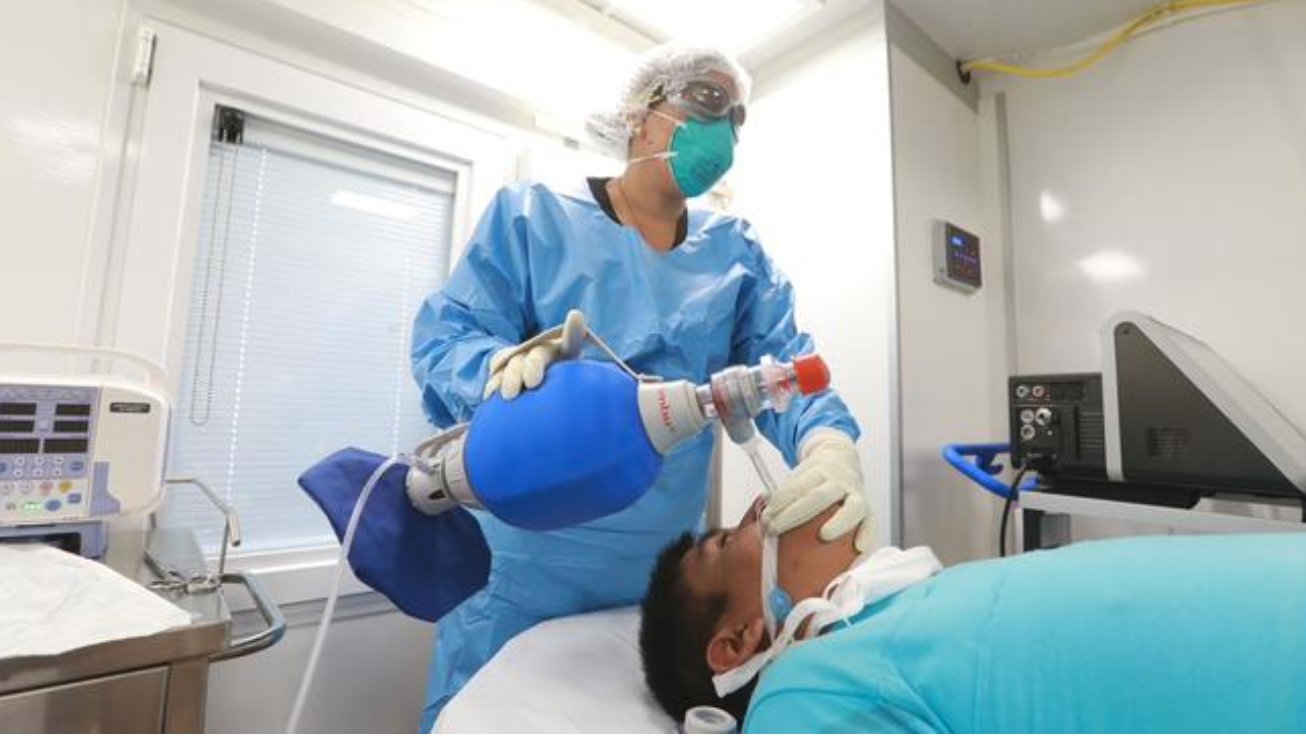
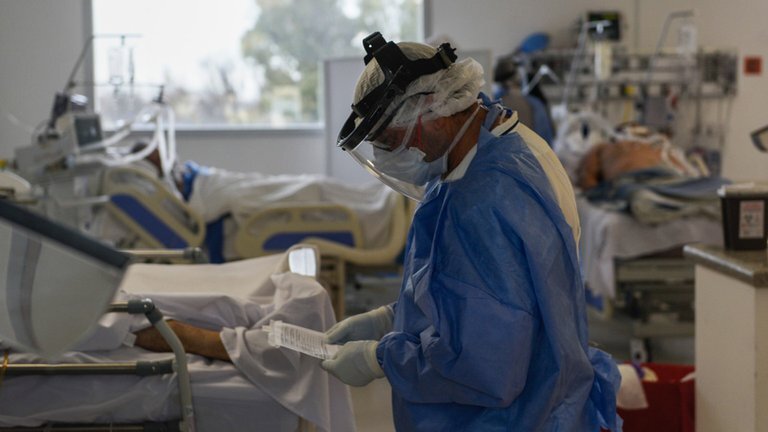
Minsa aprueba documento técnico que establece el uso de determinados fármacos en tratamiento de personas afectadas por Covid-19
31 de marzo de 2020 - 2:51 p. m.
El Ministerio de Salud aprobó la Resolución Ministerial Nº 139-2020/MINSA que adjunta el Documento Técnico: Prevención y Atención de Personas Afectadas por Covid-19 en el Perú, que establece acciones dirigidas a la prevención, diagnóstico y sobre todo, tratamiento de las personas afectadas.
Este dispositivo legal, refrendado por el titular de Salud, Víctor Zamora, toma en cuenta que si bien no hay tratamientos específicos dirigidos a pacientes con sospecha o confirmación de infección por el virus del Covid-19, se informa que existen estudios internacionales sobre el uso de fármacos en atención de pacientes Covid-19 que ofrecen un limitado nivel de evidencia.
Estos medicamentos son: Cloroquina, Hidroxicloroquina, Azitromicina, Lopinavir/ritonavir, entre otros. Y su uso solo será para el manejo de casos moderados y severos (solo pacientes hospitalizados).
El Minsa, a solicitud de las Sociedades Científicas Nacionales y en consenso con el Grupo de Trabajo Covid-19, acoge la propuesta de poner a consideración de los médicos especialistas tratantes, sobre la base de una evaluación individual del paciente y previo consentimiento informado, los esquemas de tratamiento que las Sociedades Científicas Nacionales sugieren.
El documento técnico encarga a la Dirección General de Intervenciones Estratégicas en Salud Pública la supervisión, monitoreo y difusión de lo dispuesto.
Gratuidad del tratamiento
La norma también establece los criterios técnicos y procedimientos para la prevención, diagnóstico y tratamiento de los pacientes con el nuevo coronavirus en un escenario de transmisión comunitaria, que es de aplicación obligatoria en las Instituciones Prestadoras de Servicios de Salud (IPRESS) o establecimientos de salud públicos del Minsa, a través de las Direcciones de Redes Integradas de Salud (Diris), Direcciones Regionales de Salud (Diresa) o Gerencias Regionales de Salud (Geresa), Seguro Social de Salud (EsSalud), de las Sanidades de las Fuerzas Armadas y de la Policía Nacional del Perú, así como de las IPRESS privadas.
Señala que estas instituciones públicas y privadas deben notificar la enfermedad de manera obligatoria, de acuerdo a la norma vigente emitida por el Centro Nacional de Epidemiología, Prevención y Control de Enfermedades del Minsa. En tanto, en todos los establecimientos de salud del ámbito nacional se garantiza la gratuidad en el diagnóstico, detección y tratamiento, en el marco del Aseguramiento Universal en Salud.
La medida normativa también brinda características principales del Covid-19, signos de alarma, factores de riesgo de la enfermedad, clasificación clínica (casos leve, moderado y severo), así como la evaluación y manejo de pacientes según dicha clasificación, con y sin factores de riesgo.
Protección del personal de Salud
El documento técnico establece que todo trabajador de sector salud deberá estar vacunado contra influenza estacional y neumococo, y se realizará la prueba rápida en caso de que presente síntomas respiratorios compatibles con Infección Respiratoria Aguda (IRA) o sea contacto de un caso sospechoso o confirmado de Covid-19, o haya participado directamente en la atención de estos pacientes.
Recomienda que los establecimientos implementen áreas administrativas y asistenciales diferenciadas para la atención de casos de Covid-19, con el objetivo de reducir la exposición a los trabajadores de salud. Y especifica el tipo de Equipo de Protección Personal con el que debe contar el personal de salud.
Responsabilidades
Este documento técnico delega responsabilidades específicas. La Dirección General de Intervenciones Estratégicas en Salud Pública (Dgiesp) se encargará de elaborar y proponer documentos normativos, brindar asistencia técnica y capacitación a nivel nacional, implementar y supervisar el seguimiento clínico a distancia y presencial de personas en aislamiento domiciliario y cuarentena, así como garantizar el abastecimiento y la distribución de medicamentos e insumos médicos para el diagnóstico y la atención de pacientes Covid-19, lo que incluyen los Equipos de Protección Personal y pruebas de laboratorio.
Los hospitales e institutos, a su vez, ejecutarán las normas y procedimientos técnicos dispuestos, organizarán la atención en áreas especializadas destinadas para los pacientes con Covid-19, desde los casos moderados hasta los severos. También deberán elaborar e implementar el Plan de Control de Infecciones y de Bioseguridad, asimismo garantizar el abastecimiento de medicamentos y insumos médicos para una atención más óptima y eficiente.
Además, precisa las funciones del Laboratorio de Referencia Nacional de Virus Respiratorios del Instituto Nacional de Salud (INS), encargado de conducir la red de laboratorios en el ámbito nacional. Este realizará la transferencia tecnológica sobre los métodos de diagnóstico, supervisará el control de calidad de pruebas rápidas y consolidará la información del país relativa a exámenes realizados por los Laboratorios de Referencia Regional (LRR).
El documento deja sin efecto la Resolución Ministerial Nº 084-2020/MINSA, que aprobaba el Documento Técnico: Atención y Manejo Clínico de Casos Covid-19, escenario de transmisión focalizada.
Covid-19: India Recommends Hydroxychloroquine As Prophylaxis For Healthcare Providers, Patient Family Members
The National Task force for COVID-19, constituted by Indian Council of Medical Research, has recommended the use of hydroxy- chloroquine as prophylaxis (preventive drug) of SARS-COV-2 infection for high risk population.
Chloroquine, or hydroxychloroquine, has been used to treat malaria since 1944. It can be given before exposure to malaria to prevent infection, and it can also be given as treatment afterward.Malaria is a disease that is caused by a parasite, unlike COVID-19. Nevertheless, laboratory studies show chloroquine is effective at preventing as well as treating the virus that causes severe acute respiratory syndrome, or SARS, a close cousin of COVID-19
The advisory provides for placing the following high risk population under chemoprophylaxis with hydroxy chloroquine:
• Asymptomatic Healthcare Workers involved in the care of suspected or confirmed cases of COVID
• Asymptomatic household contacts of laboratory confirmed cases
The protocol recommended by the National Task force also has been approved by the Drug Controller General of India for restricted use in emergency situations.
Along with the recommendation, the health ministry also provided a set of guidelines
1) The placing of healthcare workers under chemoprophylaxis should not instill a sense of false security. They should follow all prescribed public health measures such as frequent washing of hands, respiratory etiquettes, keeping a distance of minimum 1m and use of Personal protective equipment (wherever applicable).
2) They should self-monitor their health and report to health authorities immediately in the event of them becoming symptomatic.
3) The high risk contacts of a positive case placed under chemo prophylaxis, should remain in home quarantine while on prophylactic therapy.
4) As recommended by the said Task Force, the drug should only be given on the prescription of a registered medical practitioner. The contraindications mentioned in the recommendations should strictly be followed.
5) Apart from the symptoms of COVID-19 (fever, cough, breathing difficulty), if the person on chemoprophylaxis develops any other symptoms, he should immediately seek medical treatment of the medical practitioner who has prescribed the chemoprophylaxis.
Hydroxy-chloroquine has been found to be effective against coronavirus in laboratory studies and in-vivo studies. Its use in prophylaxis is derived from available evidence of benefit as treatment and supported by pre-clinical data.
The ministry said that recommendation for the use of hydroxy-chloroquine as a prophylactic agent against SARS-CoV-2 infection is based on these considerations, as well as risk-benefit consideration, under exceptional circumstances that call for the protection of high-risk individuals.
Source: AP Photo/Andrew Harnik
The Worldwide Lockdown May Be the Greatest Mistake in History
The idea that the worldwide lockdown of virtually every country other than Sweden may have been an enormous mistake strikes many -- including world leaders; most scientists, especially health officials, doctors and epidemiologists; those who work in major news media; opinion writers in those media; and the hundreds of millions, if not billions, of people who put their faith in these people -- as so preposterous as to be immoral. Timothy Egan of The New York Times described Republicans who wish to enable their states to open up as "the party of death."
That's the way it is today on planet Earth, where deceit, cowardice and immaturity now dominate almost all societies because the elites are deceitful, cowardly and immature.
But for those open to reading thoughts they may differ with, here is the case for why the worldwide lockdown is not only a mistake but also, possibly, the worst mistake the world has ever made. And for those intellectually challenged by the English language and/or logic, "mistake" and "evil" are not synonyms. The lockdown is a mistake; the Holocaust, slavery, communism, fascism, etc., were evils. Massive mistakes are made by arrogant fools; massive evils are committed by evil people.
The forcible prevention of Americans from doing anything except what politicians deem "essential" has led to the worst economy in American history since the Great Depression of the 1930s. It is panic and hysteria, not the coronavirus, that created this catastrophe. And the consequences in much of the world will be more horrible than in America.
The United Nations World Food Programme, or the WFP, states that by the end of the year, more than 260 million people will face starvation -- double last year's figures. According to WFP director David Beasley on April 21: "We could be looking at famine in about three dozen countries. ... There is also a real danger that more people could potentially die from the economic impact of COVID-19 than from the virus itself" (italics added).
That would be enough to characterize the worldwide lockdown as a deathly error. But there is much more. If global GDP declines by 5%, another 147 million people could be plunged into extreme poverty, according to the International Food Policy Research Institute.
Foreign Policy magazine reports that, according to the International Monetary Fund, the global economy will shrink by 3% in 2020, marking the biggest downturn since the Great Depression, and the U.S., the eurozone and Japan will contract by 5.9%, 7.5% and 5.2%, respectively. Meanwhile, across South Asia, as of a month ago, tens of millions were already "struggling to put food on the table." Again, all because of the lockdowns, not the virus.
In one particularly incomprehensible act, the government of India, a poor country of 1.3 billion people, locked down its people. As Quartz India reported on April 22, "Coronavirus has killed only around 700 Indians ... a small number still compared to the 450,000 TB and 10,000-odd malaria deaths recorded every year."
One of the thousands of unpaid garment workers protesting the lockdown in Bangladesh understands the situation better than almost any health official in the world: "We are starving. If we don't have food in our stomach, what's the use of observing this lockdown?" But concern for that Bangladeshi worker among the world's elites seems nonexistent.
The lockdown is "possibly even more catastrophic (than the virus) in its outcome: the collapse of global food-supply systems and widespread human starvation" (italics added). That was published in the left-wing The Nation, which, nevertheless, enthusiastically supports lockdowns. But the American left cares as much about the millions of non-Americans reduced to hunger and starvation because of the lockdown as it does about the people of upstate New York who have no incomes, despite the minuscule number of coronavirus deaths there. Or about the citizens of Oregon, whose governor has just announced the state will remain locked down until July 6. As of this writing, a total of 109 people have died of the coronavirus in Oregon.
An example of how disinterested the left is in worldwide suffering is made abundantly clear in a front-page "prayer" by a left-wing Christian in the current issue of The Nation: "May we who are merely inconvenienced remember those whose lives are at stake."
"Merely inconvenienced" is how the Rev. Dr. William J. Barber II, a Protestant minister and president of the North Carolina NAACP, describes the tens of millions of Americans rendered destitute, not to mention the hundreds of millions around the world rendered not only penniless but hungry. The truth is, like most of the elites, it is Barber who is "merely inconvenienced." Indeed, the American battle today is between the merely inconvenienced and the rest of America.
Michael Levitt, professor of structural biology at Stanford Medical School and winner of the 2013 Nobel Prize in chemistry, recently stated, "There is no doubt in my mind that when we come to look back on this, the damage done by lockdown will exceed any saving of lives by a huge factor."
To the left, anyone who questions the lockdown is driven by preference for money over lives. Typical of the left's moral shallowness is this headline on Salon this week:
"It's Time To Reject the Gods of Commerce: America Is a Society, Not an 'Economy,'" with the subhead reading, "America Is About People, Not Profit Margins."
And, of course, to smug editors and writers of The Atlantic, in article after repetitive article, the fault lies not with the lockdown but with President Donald Trump. The most popular article in The Atlantic this week is titled "The Rest of the World Is Laughing at Trump." The elites can afford to laugh at whatever they want. Meanwhile, the less fortunate -- that is, most people -- are crying.
Editor's Note: Want to support Townhall so we can keep telling the truth about China and the virus they unleashed on the world? Join Townhall VIP and use the promo code WUHAN to get 25% off VIP membership!
Dennis Prager is a nationally syndicated radio talk-show host and columnist. His latest book, published by Regnery in May 2019, is "The Rational Bible," a commentary on the book of Genesis. His film, "No Safe Spaces," came to theaters fall 2019. He is the founder of Prager University and may be contacted at dennisprager.com.
Doctors and Patients Are Pawns in a Dangerous Political Brinkmanship
Front line physicians treating patients with SARS-CoV2/COVID-19 are seeing an unprecedented, alarming, and escalating orchestrated attack on chloroquine (CQ) and its safer derivative, hydroxychloroquine (HCQ). Both medicines have been safely used in millions of patients worldwide for malaria prevention and treatment since they were FDA approved for safety and effectiveness in 1934 and 1955, respectively. The World Health Organization (WHO) lists CQ and HCQ as “essential medicines” because of safety, effectiveness, low cost, and wide availability.
The United States CDC itself has published guidelines on its website: “Who can take hydroxychloroquine (Placquenil)? Hydroxychloroquine can be prescribed to adults and children of all ages. It can also be safely taken by pregnant women and nursing mothers.”
The CDC Guidelines address side effects: Hydroxychloroquine is a relatively well tolerated medicine. The most common adverse reactions reported are stomach pain, nausea, vomiting, and headache. These side effects can often be lessened by taking hydroxychloroquine with food. Hydroxychloroquine may also cause itching in some people. All medicines may have some side effects. Minor side effects such as nausea, occasional vomiting, or diarrhea usually do not require stopping the antimalarial drug.”
CDC addresses duration of use: “How long is it safe to use hydroxychloroquine? CDC has no limits on the use of hydroxychloroquine for the prevention of malaria. When hydroxychloroquine is used at higher doses for many years, a rare eye condition called retinopathy has occurred. People who take hydroxychloroquine for more than five years should get regular eye exams.“
NOTICE: The CDC guidelines for use in malaria do not even mention the “fatal heart arrhythmia” hyped in the fear-mongering articles in the media recently. Rheumatology guidelines for using HCQ for Lupus and Rheumatoid Arthritis (RA) patients do not require a baseline EKG to check heart rhythm, though doctors might order one before using these medicines if needed for a patient with heart disease.
So our own CDC has said CQ and HCQ are safe and well tolerated for potentially long periods of time preventing and treating malaria. FDA later approved HCQ for treating Lupus and RA, with millions taking much higher doses over decades, not days.
Successful treatment protocols worldwide describe low dose, short duration (5-7 days) treatment early in COVID-19 to help reduce severity, rate of spread and need for hospitalization. Why the firestorm against HCQ? It simply makes no medical sense.
We DO have data from 2002 showing HCQ has potent antiviral action early in the illness of SARS-CoV. Yet CDC, FDA, and Dr. Fauci seem either ignorant of these early peer-reviewed studies, or are willfully ignoring them.
Either way, Dr. Fauci, The FDA and CDC failure to allow, and even encourage, physicians to offer HCQ as an option to COVID-19 patients early in the disease is causing serious harm to Americans in higher US death rates compared to countries using HCQ at the onset of infection. And the US has higher complication rates, longer hospitalizations, and devastating economic damage from the prolonged shutdown.
We know that Dr. Fauci, the FDA and CDA have up to the minute, country by country data on number of cases, number of deaths, and number of deaths per million in population.
It doesn’t take a rocket scientist or an MD or PhD degree to see the stark difference. U.S. politicians and entrenched bureaucrats dangerous interference in the physician-patient relationship are responsible for our higher death rates by preventing doctors from doing their jobs for patients early in the disease when the drug is most effective.
Doctors want to help patients, and save lives. They do not want their hands tied by politicians. Doctors and nurses simply want the option of taking HCQ to help keep from getting sick so they can continue to treat patients.
Why are our doctors and nurses being denied prophylactic treatment readily available and protecting healthcare workers in other countries, and even threatened with loss of their medical license if they prescribe it?
Patients with common sense, who can easily find out information about HCQ, are pleading for the opportunity to work with their physician they trust and be able to try an FDA-approved medicine in this new crisis.
Our FDA, CDC, Governors and State Medical Boards are making it appear that “off-label” prescriptions for HCQ in COVID-19 is illegal or fraudulent. Off-label simply means a new disease use different from the one originally approved.
Why is the FDA now over-ruling its own long standing regulations that allow doctors to legally prescribe any approved medicine for a new use?
Physicians have used approved medications off-label since the FDA was formed under Democrat President Franklin Delano Roosevelt at the time of World War II. Until this COVID-19 crisis, there has never been an outcry about doctors using old medicines for new uses. This freedom has allowed more rapid discovery of life-saving treatments. For example:
Amitriptyline (Elavil®), first approved for depression, is now used for nerve pain. Trazodone, approved for depression, is widely used at lower doses for sleep. Sildenafil (Viagra®), approved for erectile dysfunction, is also used to treat pulmonary artery hypertension. Dr. Fauci has failed to report any of the many positive basic science and successful clinical outcomes results from the United States and more than a dozen other countries. Why? Surely he knows the data. Why does he continue to dismiss them? He must know the clearly lower death rates in other countries using HCQ early in COVID-19.
Dr. Fauci is on record saying that we need to wait for a vaccine to safely re-open the country, yet he better than anyone knows the safety issues of vaccines rushed to market without adequate testing, and he knows they take months to years.
Political brinkmanship and hidden agendas⏤political, financial or both⏤are a dangerous and deadly game. It is time for doctors, nurses, and citizens to fight back with massive public pressure on state officials and legal actions against agencies working against the public interest.
HCQ is not a panacea, but it IS saving lives around the world⏤in countries with less sophisticated medical infrastructure and capability than we have in America.
We don’t have time to waste. People are dying. Our economy is dying.
Professor Raoult Comments on the Pandemic Evolution, the Disease Phases, the Available Treatments and … Media Delirium
In the interview, Professor Didier Raoult, ranked number 1 among the world’s infectious disease experts, comments on the media treatment of the early treatment he recommends.
“Hydroxychloroquine, chloroquine, these are incredibly used drugs … I don’t even know how the media could have gone mad around this story. It is a real madness disconnected from reality … It was just needed to speak to any doctor to know that the plaquenil is harmless … All of a sudden, we learn it’s a toxic product . It is appalling, it is completely delusional … We must return to reason, reason must regain its rights, and we must stop considering that this drug, which was discovered 80 years ago, and which has been used since, became toxic in 2020.”
For Professor Didier Raoult, the majority of doctors reacted very well to the need to treat the disease. However, concerning doctors who do not intervene quickly and let the disease progress without treating it, he says that it goes against the principles of medical practice since … Hippocrates.
Professor Raoult also commented on treatments in intensive care units, in intensive care in France: “Something has happened that is quite remarkable, absolutely extraordinary, it is the quality of care in intensive care units.”
Evolution of the Epidemic
In this new interview, the Professor first discusses the evolution of the epidemic.
that the COVID-19 epidemic follows the typical bell curve for epidemics that epidemics are disappearing, that humanity has not disappeared because of an epidemic, and that we do not know why epidemics disappear that ecosystem phenomena, that are not understood, are at the basis of the transmissibility of viruses he presents some graphs, where we can see the bell shape for Italy, Turkey, Germany, France he cites a study officially recognized in France which predicts that 99% of cases will have occurred by around May 19 he thinks that at that time, in May, the transmissibility of the virus will be lower he doesn’t think there will be a rebound, a second wave he doesn’t think 70% of the population should be immune to control the epidemic he thinks that these last two elements are kind of virtual, that they do not emanate from facts, from actual observations
The Phases of the Disease
Professor Raoult then comments on treatments for the coronavirus. He insists that now we know this disease well, and that there are several phases.
He first gives some figures:
25,000 people who have been tested at the IHU in Marseille; positivity rates peaked at 22%, it’s now down to 5 or 6% positive; among staff, about 3% have been infected, as this is equivalent to what is happening in the general population approximately 4000 patients were followed 3300 were followed in day hospital as outpatients 630 were hospitalized 1500 strains have been identified; 1300 are under culture 1900 low dose chest scans were performed, chest scans are very important because they show lung damage in people who are apparently not symptomatic 7,500 electrocardiograms were performed and supervised by cardiologists 434 genomes have been sequenced and 309 analyzed approximately 1400 dosages of hydroxychloroquine, 500 dosages of azithromycin and 300 dosages of zinc were carried out to understand the drugs and to avoid any overdose (Important to note here the appearance of Zinc in the dosages carried out)
Treatment of the Disease
>> A Reminder of Why Early Treatment is Essential Thanks to considerable experience with treatment and a database of 3,500 patients, here are Professor Raoult’s comments.
there are several stages to the disease. the first phase, which they call the incubation period, is between when the patient catches the virus and when he is symptomatic; the only thing at that the time of this incubation period is the virus, and sometimes people don’t even realize they have the virus the next period is the clinical period. There, the patients are sick, they have a fever. It’s the most common manifestation. from a therapeutic perspective, the virus is then the target, but needs to be treated relatively early. he says it’s known for the flu for example. Tamiflu works for the first, second day of the flu, and then it doesn’t work. we know that for viral infections, it is at the beginning that it is necessary to treat, after, the response against the virus is so strong that the problem is no longer the virus gradually, there is no longer a correlation between the viral load (the number of viruses) and the severity of the disease, and in the end, there are no more viruses. so there is a real sequence / evolution for the the disease, which is first a viral disease, then it becomes both viral and an immune response, and then it’s just a disease related to the immune response once the patient is healed, there is another risk, that of pulmonary fibrosis, which can make the lung no longer work at all, even when we thought the patient was cured each phase of the disease corresponds to a therapeutic phase in order to be able to treat people at the start, I always said, you have to deal with drugs that have anti-viral efficacy and are not toxic. There should not be too significant risks compared to the expected benefits. this is one of the reasons why drugs such as Remdesivir cannot be prescribed for this because their toxicity is too great; regarding Remdesivir, you can see it very well with the study which quickly disappeared from the WHO website … (note that this randomized study, which found that “remdesivir was not associated with statistically significant clinical benefits” is now public – see our article) the toxicity of Remdesivir means that it cannot be given for a disease which at first is mild what you can do is to give harmless drugs, and the Chinese have done it too. the first study led the Chinese to decide to choose hydroxychloroquine over Remdesivir, why hydroxychloroquine? Because it is not toxic, it is cheap and it is available, which was a reasonable choice if we thought that it was necessary treat people early. then things get worse, and there it’s the immune response that becomes important. there are no more viruses. There is no longer any need for antivirals at this point. Because antivirals no longer work. There are no more viruses. the problem at this point is the immune response, even if there probably remains a place for hydroxychloroquine, which is a modulator of immunity. Hydroxychloroquine, chloroquine, these are incredibly used drugs. I do not even know how the media could have gone mad around this story. It’s real madness out of touch with reality. They are sick. It would have been simple to speak to any doctor to know that plaquenil is harmless and that people are given that. before the crisis, 1.2 million boxes of plaquenil were sold in 2019, so 36 million tablets of plaquenil. and all of a sudden, we discover that plaquenil is a toxic, appalling product – it’s completely delusional. a recent study of people using plaquenil for arthritis shows an absence of heart problem how this madness took the world is something that is mysterious always be careful: chloroquine and hydroxychloroquine, if taken in sufficient doses, you can kill you we must return to reason: it is a drug that can be used if we respect the right dosage. as for azithromycin, it is the drug that has been most prescribed for respiratory infections, including those that are viral, because often there are secondary bacterial infections luckily azithromycin is particularly effective in combination with hydroxychloroquine we have already treated more than 3000 people with this. things are going very well. there have been no medication related accidents. we must return to reason, reason must resume its rights, and we must stop considering that this drug, which was discovered 80 years ago, and which has been used since, became toxic in 2020 people ate 36 million pills, just in the cities, because I’m not talking about those from the hospital, and it would have suddenly become a deadly thing? just ask your GP. everyone has already prescribed plaquenil. it’s not possible, it’s just an implausible thing that it suddenly become a deadly thing. what we forget is that this disease (COVID-19) causes damage to the heart – myocarditis. There are fatal myocarditis. Whether or not you are taking treatment, watch out as they can cause rhythm disturbances and death. we live in an era where we seek maximum safety, and it is legitimate to look at the things that are possibly associated with torsades de pointe: potassium base, co-medications, the length of the ECG QT interval, all of these are things you can do, and if you do that, you have no trouble; all this is very simple.
The Role of Medical Doctors
Regarding the role of medical doctors today in the crisis, here are the comments of Professor Didier Raoult.
I found that generally speaking, medical doctors react very well. I think I am in tune with most of the practitioners, who see the sick and who say: listen, something has to be done. A lot of people react like this, so we give azithromycin, we give macrolides, we treat patients like that. The idea that we can leave people until they have respiratory failure without giving them anything … medicine has never done that. Medical doctors treat people. We give them something. At least to reassure them, to say that we take care of them. You can’t say: people are sick, they are left in a bed until they can’t breathe, and there they go to the hospital. This is against all principles of medical practice since … Hippocrates. We can’t do that. We can’t validate that. He comments on the situation of Paris versus that of Marseille: they use more hydroxychloroquine and azithromycin in Paris than in Marseille. Perhaps the media, but Parisian doctors and patients, they are no crazier than the others: they want the medications. It’s a story of Parisian media. But Parisians are normal humans, and when they are sick, they want to be treated. And Parisian doctors, when their patients are sick, they want to treat them. These are humans – not aliens.
About Treatment in Intensive Care
something quite remarkable, absolutely extraordinary, has happened: the quality of care in intensive care units. I know it’s like that in Marseille. In Paris, my friends tell me the same thing. When there are these forms of respiratory distress, in general the mortality is more than 20 or 25%. In the American data series, what we see are absolutely considerable mortalities. what friends and colleagues tell us is that we have a much lower mortality: 9 or 10%. A colleague in Paris told me the same thing: 9 or 10%. this is because they have a network of intensive care specialists and they exchange information that they have as they go, and which are not yet published, in particular regarding coagulation there are big coagulation issues. They changed their therapeutic approach. They adapted it by giving anti-coagulants as soon as possible, to avoid pulmonary embolism, which can be a cause of sudden death. they also used “outside protocol” medication to control the immune response. when this immune response goes crazy, you have to control it, and there are drugs for that; such drugs should be used, even if there are no randomized studies, because this is about saving the lives of people in a situation that is compassionate; these tools must be used: when you have such severe situations, you have to use whatever you can to save people’s lives this approach there and the French ICUs amount to an incredible reaction, because the ICUs were overflowed, there are units that were installed in conditions that are almost war conditions – yet the result is quite exceptional. we could have had 30% more deaths, if it had not been for the quality of care in the ICUs. I think that is very good and that many people have been saved thanks to high quality ICU treatment. regarding the last phase, after intensive care, there are real questions, and pulmonologists will take care of that, for how to detect pulmonary fibrosis pulmonary fibrosis happened with people treated in ICU who have had this terrible inflammatory reaction, but also in other situations, which pose a real question for the future.
Coronavirus: The End of the Crisis?
Executive Summary
Hydroxychloroquine (HCQ) and Azithromycin (AZ) for the treatment of the Coronavirus (COVID-19) has received substantial attention in recent days. As described in more detail below, HCQ and AZ have very well-established safety profiles. A study from the South of France, showing the effectiveness of these drugs in the treatment of COVID-19, and the University of Washington protocols have specified the following:
Perform baseline EKG, address any cardiology risks and monitor EKG throughout entire treatment period[2] Determine patient allergies or potential for adverse reactions and contraindications to both drugs Administer 200 milligrams of Hydroxychloroquine sulfate, three (3) times per day (a total of 600 milligrams per day) for ten (10) days[3] Administer Azithromycin at 500 milligrams on day 1, followed by 250 milligrams per day for four (4) days[4] It must be emphasized that this article is written by attorneys who are reporting these treatment protocols from medical authorities.
HCQ and AZ are approved by the United States Food and Drug Administration (FDA) for diseases other than the Coronavirus.[5] Medical practitioners might be reluctant to prescribe these drugs as an off-label use given possible liability. However, medical practitioners may protect themselves through informed consent, a well-established concept under New York State law, described in detail below.
Introduction
“Hydroxychloroquine and Azithromycin, taken together, have a real chance to be one of the biggest game changers in the history of medicine.” President Donald Trump, Twitter, March 21, 2020. New York State’s Governor Andrew Cuomo announced in a televised news conference on March 22, 2020 that these drugs will be the subject of clinical trials in New York State starting March 24, 2020.[6]
Treatment Summary
The utilization of Hydroxychloroquine (HCQ) and Azithromycin (AZ) as a treatment for the coronavirus (COVID-19) is the subject of a study from the south of France issued March 18, 2020.[7] We understand that this study will be published in the International Journal of Antimicrobial Agents.[8] A copy of the study is attached. The study was performed under the direction of Didier Raoult, MD, PhD and Philippe Gautret, MD, PhD, both widely recognized infectious disease specialists.
In this study, HCQ and AZ were utilized to treat COVID-19 patients. The study involved 36 patients, which included 16 patients in a control group. The results showed patients prescribed both drugs were 100% virologically cured of COVID-19 within six (6) days. Patients that were not treated (control group) did not show significant improvement. As the authors of this study explained, “our study has some limitations including a small sample size…”. There were 20 patients that were treated: six (6) patients treated with HCQ and AZ, that resulted in the 100% virological cure and 12 patients treated with only HCQ, that showed substantial improvement but not as significant as when both drugs were used. Obviously, this is a small group, but the results appear promising. The authors ultimately “recommend that COVID-19 patients use [HCQ and AZ] to cure their infection and to limit the transmission of the virus...”.
Interestingly, the authors state these compounds could be useful “to prevent the transmission of the virus, especially for healthcare workers.” This raises the prospect for a preventative therapy. Didier Raoult MD, PhD and his team stated that, “[w]e think that it is not moral that this ... is not systematically included in the therapeutic trials concerning the treatment of Covid-19 infection ...”.[9] Additionally, Didier Raoult, MD, PhD and Philippe Gautret, MD, PhD have suggested that:
(1) individuals without symptoms who test positive for COVID-19 should be treated promptly with HCQ and AZ to limit or avoid symptoms and reduce the spread of infection,
(2) if the symptoms have already resulted in significant respiratory issues, such as permanent lung damage, it is unlikely HCQ and AZ would be able to reverse this damage, and
(3) it is important to test everyone as soon as possible, especially high-risk healthcare workers, and treat immediately before symptoms arise.[10]
Such a strategy would require increased tracking and testing for COVID-19. We understand that in China, and perhaps in South Korea, cell phones were used to trace people who were in recent contact with COVID-19 patients as a means to ensure thorough tracking and containment of the virus.
There is anecdotal information suggesting HCQ is currently part of the protocol for treating COVID-19 patients in South Korea and China and that hospitals in the United States are using HCQ for the treatment of COVID-19 patients. Daniel Dae Kim, a well-known actor from South Korea, recently shared his COVID-19 experience on social media, including his use of HCQ and AZ as treatment.
Based on the treatment procedure in the study described above and guidance compiled from hospitals like the University of Washington in Seattle[11], the following is a summary of treatment procedures (please consider that we are attorneys, not medical professionals, and are reporting from medical authorities):
Perform baseline EKG and address any cardiology risks and monitor EKG throughout entire treatment period[12] Determine patient allergies or potential for adverse reactions and contraindications to both drugs Administer 200 milligrams of Hydroxychloroquine sulfate, three (3) times per day (a total of 600 milligrams per day) for ten (10) days[13] Administer Azithromycin at 500 milligrams on day 1, followed by 250 milligrams per day for four (4) days[14]
Treatment Safety
HCQ has been widely available and used in medicine since 1955.[15] The primary approved uses of HCQ are for treatment of malaria, lupus and rheumatoid arthritis.[16] The safety profile of HCQ has become established over decades of learning and understanding the risks and side effects associated with its use alone and in combination with other drugs.
Similarly, AZ has a safety profile that has been established since its use beginning in 1991.[17] The most commonly known use of AZ is Zithromax or Z-Pak, which is approved for use to treat acute bacterial exacerbations of chronic obstructive pulmonary disease, acute bacterial sinusitis, community-acquired pneumonia, pharyngitis/tonsillitis, uncomplicated skin and skin structure infections, urethritis and cervicitis and genital ulcer disease.[18]
Having a thorough understanding of the safety profiles of both of these drugs is crucial to the acceptance of their use as a potential treatment of COVID-19. Fortunately, we have decades of studies demonstrating the safety of these drugs, including one such study which states “[b]oth azithromycin and chloroquine have been safely administered individually in all trimesters of pregnancy.” The report concludes that “[t]he combination may be safely administered any time during pregnancy…”.[19] While these studies use chloroquine, rather than HCQ, it is understood that HCQ has a better safety profile than chloroquine.[20]
Legal Implications for Use of HCQ and AZ as Off-Label Drugs
In addition to the approval and marketing of prescription drugs in the United States, the Food and Drug Administration (FDA) also oversees labeling. All prescription drugs available in the United States today have their own FDA-approved label which contains important information including, among many others, the approved uses of the drug. When a medical practitioner prescribes the drug for a use that is not specified on the FDA-approved label it is considered an "off-label” use.
Currently, there is no authority limiting medical practitioners from prescribing off-label prescription drugs. The Agency for Healthcare and Research Quality, the lead Federal agency charged with improving the safety and quality of America's health care system, states that as many as one in five prescriptions written today are for an off-label use and that the practice is “legal and common.”[21] Indeed, the FDA website currently states, “[f]rom the FDA perspective, once the FDA approves a drug, healthcare providers generally may prescribe the drug for an unapproved use when they judge that it is medically appropriate for their patient.”[22]
A doctor’s need to prescribe drugs off-label stems from science and medicine moving faster than the FDA’s bureaucracy and regulation. The pace of medical discovery invariably runs far ahead of FDA’s regulatory machinery, and off-label use is frequently state-of-the-art treatment.[23] The editor of the Journal of the American Medical Association testified before Congress that for a product to have the most effective potential benefits, law and regulation should and must follow, not precede, science. There are too many variations in clinical circumstances and too much time delay in regulations to allow the government to impede the physician’s ability to practice in these regards when it is medically appropriate.[24]
While drug manufacturers are aware of the prevalence of off-label uses, many may lack an incentive to get approval for the off-label uses due to the expensive and lengthy FDA-approval process. With drugs that have been approved for as long as HCQ and AZ, there is little economic benefit for manufacturers to obtain FDA approval. This concept may also explain the wide use and acceptance of prescribing off-label drugs.
Despite a seemingly wide-spread use of off-label prescription drugs, medical practitioners should be confident they can be protected from potential malpractice claims. The law prescribes a number of ways a medical practitioner may seek to substantially reduce potential liability associated with prescribing off-label use prescription drugs.
Informed Consent in New York State
It is essential that a medical practitioner disclose to a patient the alternatives, risks and benefits associated with a particular treatment prior to administering such treatment. When a patient agrees to treatment, it is considered “informed consent.” The lack of informed consent can cause serious issues for a medical practitioner. New York Public Health Law § 2805-D addresses informed consent.[25] Below is a summary of New York Public Health Law § 2805-D in relevant part. A complete version of the statute is attached.
A medical practitioner must obtain informed consent in those cases involving either (a) non-emergency treatment, procedure or surgery, and (b) a diagnostic procedure which involved invasion or disruption of the integrity of the body.[26] A medical practitioner’s failure to obtain informed consent from a patient in these situations can lead to malpractice liability.
For medical practitioners, it shall be a defense to any malpractice action based upon an alleged failure to obtain such an informed consent that: (i) the patient assured the medical practitioner he/she would undergo the treatment regardless of the risk involved, or the patient assured the medical practitioner that he/she did not want to be informed of the matters to which he would be entitled to be informed; or (ii) consent by or on behalf of the patient was not reasonably possible.[27] Notably, under New York law, a medical practitioner likely need not obtain informed consent in an emergency. [28]
Thus, prior to prescribing HCQ and AZ, or any off-label use medication, medical practitioners should be certain that their patients are fully informed of the risks associated with the proposed treatment. Medical practitioners can prove informed consent was obtained prior to the administration of an off-label treatment in the following ways:
Provide the patient with ample written literature on the drugs in a format that can be easily read and comprehended by the patient. Written literature should be available in a variety of languages. Have the patient provide an ink signature on a form wherein the patient acknowledges that the use of HCQ and AZ in the treatment of COVID-19 is an off-label use and states that the patient fully understands the procedure, as well as, the potential for risks, benefits, side effects and alternatives (including no treatment) associated with the treatment and authorizes the medical practitioner to proceed with such treatment. The form itself should stat the treatment procedure, as well as, the potential for risks, benefits, side effects and alternatives specific to the individual patent. Have the treating medical practitioner engage in an oral conversation with the patient about the treatment wherein the potential risks, benefits, side effects and alternatives are thoroughly explained to the patient in a manner that is simple enough for the patient to understand. Also, allow the patient to: (1) ask questions to his/her satisfaction and (2) confer with family advisors, if desired. Medical practitioners should seek to have a witness present during this discussion, as well as, a translator if necessary.
Assumption of Risk Defense in New York State
New York law recognizes assumption of risk defense in negligence tort liability actions. This can also be applicable in a malpractice case. Assumption of risk can be expressed or implied. The New York Court of Appeals stated that, “[e]xpress assumption, which was held to preclude any recovery, resulted from agreement in advance that [medical practitioner] need not use reasonable care for the benefit of [patient] and would not be liable for the consequence of conduct that would otherwise be negligent.”[29] “Implied assumption was founded not on express contract, but on plaintiff's voluntarily encountering the risk of harm from defendant's conduct with full understanding of the possible harm to himself or herself.”[30]
The Court of Appeals for the Second Circuit has stated, “[e]xpress assumption of risk is a total bar to recovery.”[31] Quoting an earlier Second Circuit decision, the Court states, “[i]n Schneider, we stated that "[w]hile a patient should be encouraged to exercise care for his own safety, we believe that an informed decision to avoid surgery and conventional chemotherapy is within the patient's right `to determine what shall be done with his own body.'" 817 F.2d at 995 (citations omitted). This conclusion led us to hold that a patient may expressly assume the risk of malpractice and dissolve the physician's duty to treat a patient according to the medical community's accepted standards.”[32]
The bottom line is that “[t]he standard for proving negligence in a malpractice case is whether the treatment deviates from accepted medical standards.”[33] However, even when deviating from accepted medical standards, medical practitioners can substantially limit or potentially eliminate their liability. Thus, is it critical that medical practitioners use discretion in their decision to administer HCL and AZ and as to the sufficiency of their disclosure and informed consent practices with their patients. This practice should be applied to the prescription of all off-label uses of drugs.
Conclusion
As we continue into this unchartered territory that is COVID-19, we are hopeful that medical practitioners can rest assured of the legal protections in place that are associated with the prescription of an off-label drug use. Medical practitioners should use their discretion on a case by case basis, but the objective is that they should not feel inhibited by what might be considered an off-label use stigma. These unprecedented times call for unprecedented actions, but we must still be logical and rational in our actions.
Please do not regard this paper as medical or legal advice. The authors of this article are not medical practitioners. Appropriate legal and medical professionals should be consulted for each specific situation.
Breaking News: ICMR Permits Prophylactic Use Of Hydroxy—Chloroquine For Coronavirus; Check Out Indications, Dose
New Delhi: .The Indian Council of Medical Research (ICMR), the apex medical research body spearheading India's battle against coronavirus has now released an Advisory on the use of hydroxy—chloroquine as prophylaxis for SARS-CoV-2 infection This came after the National Task force for COVID-19 constituted by Indian Council of Medical Research recommends the use of hydroxy— chloroquine for prophylaxis of SARS-CoV-2 infection for high risk population.
The Advisory provides for placing the following high risk population under chemoprophylaxis with hydroxy chloroquine Asymptomatic Healthcare Workers involved in the care of suspected or confirmed cases of COVID-19 Asymptomatic household contacts of laboratory confirmed cases The protocol recommended by the National Task force has been approved by the Drug Controller General of India for restricted use in emergency situations.
FDA approves compassionate use of anti-malaria drug chloroquine for the treatment of coronavirus
Stephen Hahn, commissioner of the Food and Drug Administration (FDA), said that allowing COVID-19 patients the “right to try” would provide the FDA with additional data on the effectiveness of the drugs against coronavirus, potentially speeding up its release to the public. Hahn said drugs like remdesivir are “going through the normal process,” but will be made available for “compassionate use,” allowing doctors to have emergency access to the drugs if requested.
The idea behind the compassionate use is not new. It is a longstanding FDA program that allows for a physician to use an investigational drug in a patient under a protocol that undergoes review by an institutional review board and the FDA itself, while in addition enabling the agency to collect data.
The announcement came during the daily Coronavirus Task Force press briefing. Below is a video of U.S. President Trump making the announcement.
Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020 FDA issued an Emergency Use Authorization (EUA) to allow hydroxychloroquine.
The U.S. Food and Drug Administration today announced the following actions taken in its ongoing response effort to the COVID-19 pandemic:
On March 28, 2020, the FDA issued an Emergency Use Authorization (EUA) to allow hydroxychloroquine sulfate and chloroquine phosphate products donated to the Strategic National Stockpile(SNS) to be distributed and used for certain hospitalized patients with COVID-19. These drugs will be distributed from the SNS to states for doctors to prescribe to adolescent and adult patients hospitalized with COVID-19, as appropriate, when a clinical trial is not available or feasible. The EUA requires that fact sheets that provide important information about using chloroquine phosphate and hydroxychloroquine sulfate in treating COVID-19 be made available to health care providers and patients, including the known risks and drug interactions. The SNS, managed by ASPR, will work with the Federal Emergency Management Agency (FEMA) to ship donated doses to states.
On March 29, 2020, the FDA issued an immediately in effect guidance that outlines an enforcement policy to help expand the availability and capability of sterilizers, disinfectant devices and air purifiers. The devices include those intended to make devices sterile, kill pathogens or other microorganisms and kill pathogens or microorganisms in the air. This policy reflects FDA’s commitment to ease burdens on health care providers and facilities as they face COVID-19.
The FDA amended the Emergency Use Authorization (EUA) for the Battelle Decontamination System for use in decontaminating compatible N95 respirators for reuse by health care personnel during the COVID-19 pandemic. This EUA is an important step forward in helping to reduce shortages in critical N95 respirators, by allowing for these important devices, when decontaminated, to be reused by medical professionals on the front lines of the COVID-19 pandemic.
On March 30, 2020, the FDA issued an immediately in effect guidance to help expand the availability of surgical apparel for health care professionals, including gowns (togas), hoods, and surgeon’s and patient examination gloves during this public health emergency.
FDA and FTC issued warning letters to two companies for selling unapproved products claiming to mitigate, prevent, treat, diagnose or cure COVID-19. One of the companies, Corona-cure.com, was warned for selling the product Coronavirus Infection Prevention Nasal Spray with misleading claims on its website that its product is safe and/or effective for the treatment or prevention of COVID-19. The agencies also warned Carahealth for selling its herbal products, including “Carahealth Immune,” with misleading claims of prevention and/or treatment of COVID-19. We are particularly concerned that unapproved drugs that claim to cure, treat, or prevent serious conditions may cause consumers to delay or stop appropriate medical treatment, leading to serious or life-threatening harm. There is currently no approved treatment or preventative measure for COVID-19. FDA and FTC are closely monitoring social media, the online marketplace, and incoming reports for fraudulent COVID-19 products on the market.
The FDA issued an updated guidance, “Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic,” with an appendix adding questions and answers on this subject. We plan to update this appendix as new questions arise. This guidance is intended for industry, investigators and institutional review boards and was issued because we recognize that the COVID-19 pandemic may impact the conduct of clinical trials of medical products, including drugs, devices and biological products.
Diagnostics update to date: During the COVID-19 pandemic, the FDA has worked with more than 230 test developers who have said they will be submitting emergency use authorizations (EUA) requests to FDA for tests that detect the virus. To date, 20 emergency use authorizations have been issued for diagnostic tests, including Abbott Diagnostics Scarborough, Inc., ID NOW COVID-19, a rapid (13 minutes or less) test. Additionally, the FDA has been notified that more than 110 laboratories have begun testing under the policies set forth in our COVID-19 Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency Guidance. The FDA also continues to keep its COVID-19 Diagnostics FAQ up to date.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Un grupo de médicos y especialistas cuestionaron a los infectólogos que asesoran a Alberto Fernández
La agrupación Epidemiólogos Argentinos Metadisciplinarios, ocupados de estudiar desde otra visión la problemática sanitaria de la pandemia por COVID-19, le manifestaron al presidente Alberto Fernández sus inquietudes respecto de algunas medidas tomadas en la cuarentena y cuestionaron la rigidez y la extensión de la misma.
Tras casi cuatro meses de aplicación de las medidas de excepción, los especialistas advirtieron sobre la continuidad de decisiones sanitarias que se habían tomado solamente en función de una emergencia y afirmaron que con su prolongación están generando efectos perjudiciales para la salud comunitaria, en la economía doméstica y hasta en los ritos religiosos, entre otros aspectos sociales.
La agrupación, que mantienen una mirada epidemiológica alternativa, cuestionó además que se haya priorizado al equipo de infectólogos que asesora al presidente, por sobre “las experiencias y saberes establecidos en la epidemiología, la antropología, el derecho, la sociología, la psicología social y la gerontología, entre muchas otras”.
Para tratar de discernir las dudas acerca de las decisiones que se tomaron, el abordaje y tratamiento de esta epidemia, los expertos hicieron público un listado con 16 preguntas al Presidente con el objetivo de disipar esos interrogantes y la incertidumbre futura.
¿Por qué se instrumentó una cuarentena para individuos sanos cuando no hay registro de tal restricción en la historia de la humanidad? ¿Qué criterios científicos y particularmente epidemiológicos se aplicaron para extender la cuarentena total a cinco provincias sin casos y a otras seis con uno o dos casos? ¿Por qué no se le dio suficiente importancia a la producción natural de anticuerpos por vía del contagio en población no vulnerable, privilegiando la inmunidad adquirida mediante vacunas?, son algunas de las preguntas que le acercaron al Presidente.
Widely Publicized Hydroxychloroquine Study Appears To Be Based On Bogus Data (Update: Study Retracted)
This certainly looks like a significant case of medical fraud. A company called Surgisphere claimed it had a database of information gathered from over 600 hospitals around the world on the success of hydroxychloroquine for the treatment of the coronavirus. The company’s owner authored several papers published in prominent medical journals based on this data. Those papers were then widely reported in the media last month and resulted in drug trials that were already underway being paused:
SEE ALSO: Two soldiers appeared in American Samoa’s DNC roll call video – now they are being investigated
On its face, it was a major finding: Antimalarial drugs touted by the White House as possible COVID-19 treatments looked to be not just ineffective, but downright deadly. A study published on 22 May in The Lancet used hospital records procured by a little-known data analytics company called Surgisphere to conclude that coronavirus patients taking chloroquine or hydroxychloroquine were more likely to show an irregular heart rhythm—a known side effect thought to be rare—and were more likely to die in the hospital.
Within days, some large randomized trials of the drugs—the type that might prove or disprove the retrospective study’s analysis—screeched to a halt. Solidarity, the World Health Organization’s (WHO’s) megatrial of potential COVID-19 treatments, paused recruitment into its hydroxychloroquine arm, for example.
Just to emphasize how this Lancet study was greeted by the media, here’s the NY Times story about it which is headlined, “Malaria Drug Taken by Trump Is Tied to Increased Risk of Heart Problems and Death in New Study.”
Early treatment with hydroxychloroquine: a country-based analysis
Many countries either adopted or declined early treatment with HCQ, effectively forming a large trial with 1.8 billion people in the treatment group and 663 million in the control group. As of August 19, 2020, an average of 49.0/million in the treatment group have died, and 446.9/million in the control group, relative risk 0.110. After adjustments, treatment and control deaths become 99.6/million and 652.8/million, relative risk 0.15. The probability of an equal or lower relative risk occurring from random group assignments is 0.005. Accounting for predicted changes in spread, we estimate a relative risk of 0.23. The treatment group has a 77.4% lower death rate. Confounding factors affect this estimate. We
examined diabetes, obesity, hypertension, life expectancy, population density, urbanization, testing level, and intervention level, which do not account for the effect observed.